Multi-target cell and gene therapiesfor unmet medical needs
Aurealis 4-in-1 multi-target cell and gene therapy platform is the game-changing solution for complex multi-factorial diseases.
Discover how our technology is re-defining the treatment of chronic wounds (diabetic foot ulcers, venous leg ulcers), cancers (ovarian, peritoneal, bladder, recurrent melanoma), and beyond.
THE TEAM, THE SCIENCE & the outcome
THE AUREALIS TEAM
At Aurealis Therapeutics, we are passionate about advancing science to make a difference for patients suffering from chronic wounds, deadly cancers, inflammatory diseases.
Meet the Management Team, the Scientific Advisors, the Board of Directors, and the Aurealis Therapeutics Team Members who make it happen.
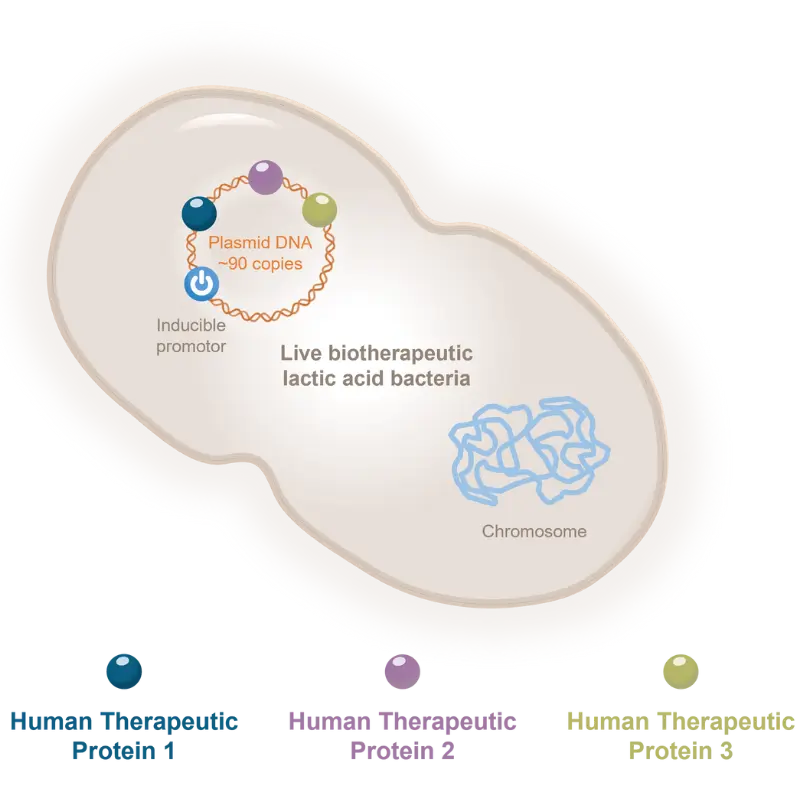
THE SCIENCE
Our team takes pride in mastering the most advanced cell and gene therapy science and applying it in real life, from the lab to the clinic.
Discover how our multi-target cell and gene therapy platform allows to modulate tissue microenvironment and tackle complex multi-factorial diseases.
THE OUTCOME
Generating pre-clinical, clinical and health-economic evidence is how we want to make a difference. Seeing non-healing wounds heal again, seeing how cancer deaths can be prevented, and ultimately improving patient outcome – this is what matters the most to us.
UPCOMING EVENTS
recent PAST EVENTS
- 20 October, 2025
- Beijing, Shanghai and Hong Kong
During the week of 20–25 October 2025, Aurealis Therapeutics’ CEO Juha Yrjänheikki and COO Laurent Décory traveled to Beijing, Shanghai and Hong Kong for a series of high-level meetings with
- 7 October, 2025
- Amsterdam, Netherlands
Aurealis Therapeutics Drug Safety Officer, Matleena Piiroinen, attended the World Drug Safety Congress Europe 2025, that took place 7–8 October in Amsterdam, The Netherlands. As Europe’s leading forum for drug
- 16 September, 2025
- Boston, USA
Aurealis Therapeutics attended LSX World Congress USA, 16-17 September 2025 in Boston. This was a leading forum that brought together innovators, investors, and senior life science dealmakers to shape the
Aurealis Therapeutics Announces Positive Blinded Evaluator Efficacy Results of “DIAMEND” Phase-2 Randomized Controlled Trial: AUP-16 Tripling the Complete Wound Closure Rate in Patients with Chronic Diabetic Foot Ulcers
Aurealis Therapeutics Announces Positive Blinded Evaluator Efficacy Results of “DIAMEND” Phase-2 Randomized Controlled Trial: AUP-16 Tripling the Complete Wound Closure Rate in Patients with Chronic Diabetic Foot Ulcers Zug Switzerland...
Aurealis Therapeutics to Attend Swiss Biotech Day 2025
Aurealis Therapeutics, a synthetic biology company developing multi-targeting, scalable, low cost of goods cell and gene therapies for high unmet medical needs, announced today that Chief Operating Officer Laurent Décory will...
Aurealis Therapeutics Concludes Largest EWMA Participation to Date with Exciting Investigator Data of DIAMEND Phase 2 DFU Clinical Trial Presented by Prof. Alberto Piaggesi
Watch highlights video here. Aurealis Therapeutics, a synthetic biology company developing multi-targeting, scalable, low cost of goods cell and gene therapies for high unmet medical needs, proudly concludes its most significant...
Aurealis Therapeutics Raises CHF 8 Million to Complete Phase 2 Clinical Studies in Chronic Wounds and Accelerate Oncology Program
Aurealis Therapeutics, a synthetic biology company developing multi-targeting, scalable, low cost of goods cell and gene therapies for high unmet medical needs, has raised CHF 8 million from existing investors...
Aurealis Therapeutics Announces Peer-Reviewed Paper Published from Phase 1 Study of AUP-16 for Non-Healing Diabetic Foot Ulcers
Aurealis Therapeutics, a clinical-stage Swiss-Finnish company developing multi-target cell and gene therapies for chronic wounds and cancer, is proud to announce the publication of its AUP-16 Phase 1 clinical trial...
Aurealis Therapeutics Announces Last Patient Last Treatment in DIAMEND Phase 2 Diabetic Foot Ulcer Clinical Trial of AUP-16
Aurealis Therapeutics, a clinical-stage Swiss-Finnish company developing multi-target cell and gene therapies for chronic wounds and cancer, is pleased to announce that the last patient in its DIAMEND AUP-16 Phase...
