Innovative treatment for Diabetic Foot Ulcers
Diabetic Foot Ulcers can heal with our multi-targeting product AUP-16
WHAT ARE DIABETIC FOOT ULCERS (DFUs)?
Diabetic Foot Ulcers (DFUs) are open sores or wounds that typically occur on the feet of people with diabetes. They represent one of the most challenging and serious complications of diabetes, particularly in those with poorly controlled blood sugar levels, neuropathy (nerve damage), and peripheral arterial disease (poor circulation). DFUs affect millions of people worldwide and can often lead to amputations or even death. Understanding the epidemiology, disease burden, and the pressing need for innovative treatments is important to understand this global health issue.
The lifetime risk of developing a DFU is 19-34% for people with diabetes. The prevalence of DFUs is estimated to be 6.3% in people with diabetes worldwide. In Europe, approximately 5 out of 100 people with diabetes will develop a foot ulcer each year, and up to 25 out of 100 people may get one at some point in their lives. In the United States, between 4.5 to 10 out of 100 people with diabetes will develop a foot ulcer at any given time. These numbers show that DFUs are a common problem in urgent need for better treatments.






BURDEN AND COST OF DIABETIC FOOT ULCERS
DFUs are a major cause of morbidity and mortality in people with diabetes. The impact of DFUs goes beyond physical pain. They greatly increase the risk for amputation, leading to a significant disability and affecting people’s quality of life. In some cases, DFUs can even lead to death.
The economic burden of DFUs is also significant for individuals and health care systems. The total global cost of DFUs to society is estimated to be between 90-100 billion US $ per year. This includes both, the direct and indirect costs. The direct costs of DFUs include money spent on hospital visits, doctor visits, surgeries and ongoing care. The indirect costs include lost productivity, caregiving, and pain and suffering.
The cost of DFU varies depending on the severity of the ulcer and the treatment required.
However, even a relatively simple ulcer can be expensive to treat e.g. the average cost of treating a non-healing DFU in the USA is close to 9,000 US $.
The indirect costs of DFUs can also be significant.
For example, a study in the United Kingdom found that the average cost of lost productivity due to a DFU was £5,000 per patient.
The total cost of DFUs is expected to increase in the coming years due to the rising prevalence of diabetes.
In 2021, there were an estimated 425 million adults diagnosed with diabetes worldwide. This number is expected to reach 578 million by 2030.
The global burden of DFUs is expected to increase in the coming years due to the rising prevalence of diabetes. There is a need for more research into the prevention and treatment of DFUs in order to reduce the associated morbidity, mortality, and economic burden.
CURRENT TREATMENTS FOR DIABETIC FOOT ULCERS
Some of the most common treatments for DFUs include:
• Debridement: This is the removal of dead tissue from the ulcer. It can be done surgically or with special dressings.
• Wound dressings: There are a number of different types of wound dressings available. They help to keep the ulcer clean and moist, which helps to promote healing.
• Offloading: This is used to help remove pressure on the ulcer due to body weight. It can be done with offloading shoes or total contact casting.
• Antibiotics: If the ulcer is infected, antibiotics may be prescribed.
• Surgery: In some cases, surgery may be necessary to remove the ulcer or to repair the damage to the foot.
Current treatments for diabetic foot ulcers face limitations that hinder the complete healing of the wound.
- Debridement: Even though removing dead tissue us crucial for wound healing, it only addresses one aspect of the problem.
- Wound dressings: Advanced dressings can create a moist environment for healing and prevent infection. However, they don’t address the root causes of the ulcer or promote healing from within.
- Offloading: This method effectively reduces pressure on the ulcer, but strict adherence can be difficult for patients, and it does not address circulatory problems that limit healing.
- Antibiotics: These are essential for fighting infections, but do not address any other factors that are essential for wound healing.
- Surgery: While surgery can remove infected tissue or improve blood flow, it is an invasive procedure with risks and may not be suitable for all patients.
AUP-16 MULTI-TARGET THERAPY FOR DIABETIC FOOT ULCERS
LEAD CLINICAL ASSET FOR CHRONIC WOUNDS
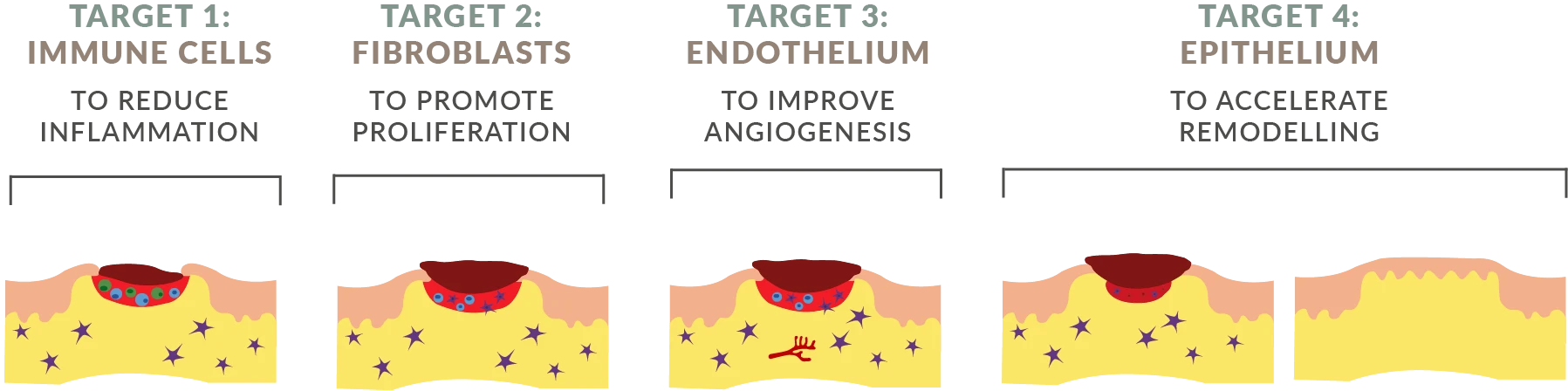
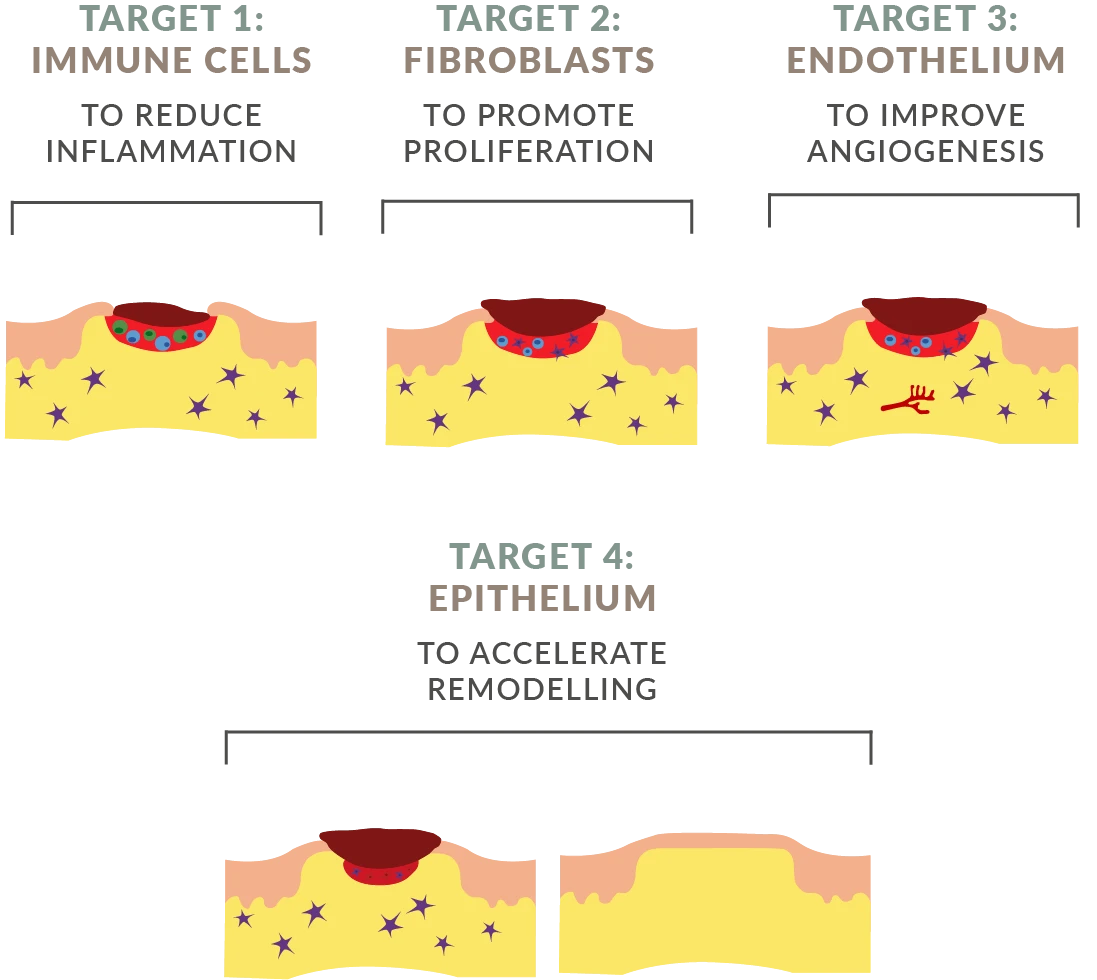
We believe that, when treating diabetic foot ulcers one needs to hit multiple targets to be disease-modifying. Very few companies or products can claim to be truly multi-target. Carefully designed and developed pharmaceutical products, such as our Advanced Therapy Medicinal Product (ATMP) AUP-16, will make the difference for patients. Our unique multi-target ATMP approach promotes the healing of the wound by promoting tissue regeneration and releasing active biological therapeutic agents within the wound itself.
AUREALIS THERAPEUTICS 4-IN-1 PRODUCT AUP-16
One Active Pharmaceutical Ingredient (API) product with the benefits of four therapeutics.
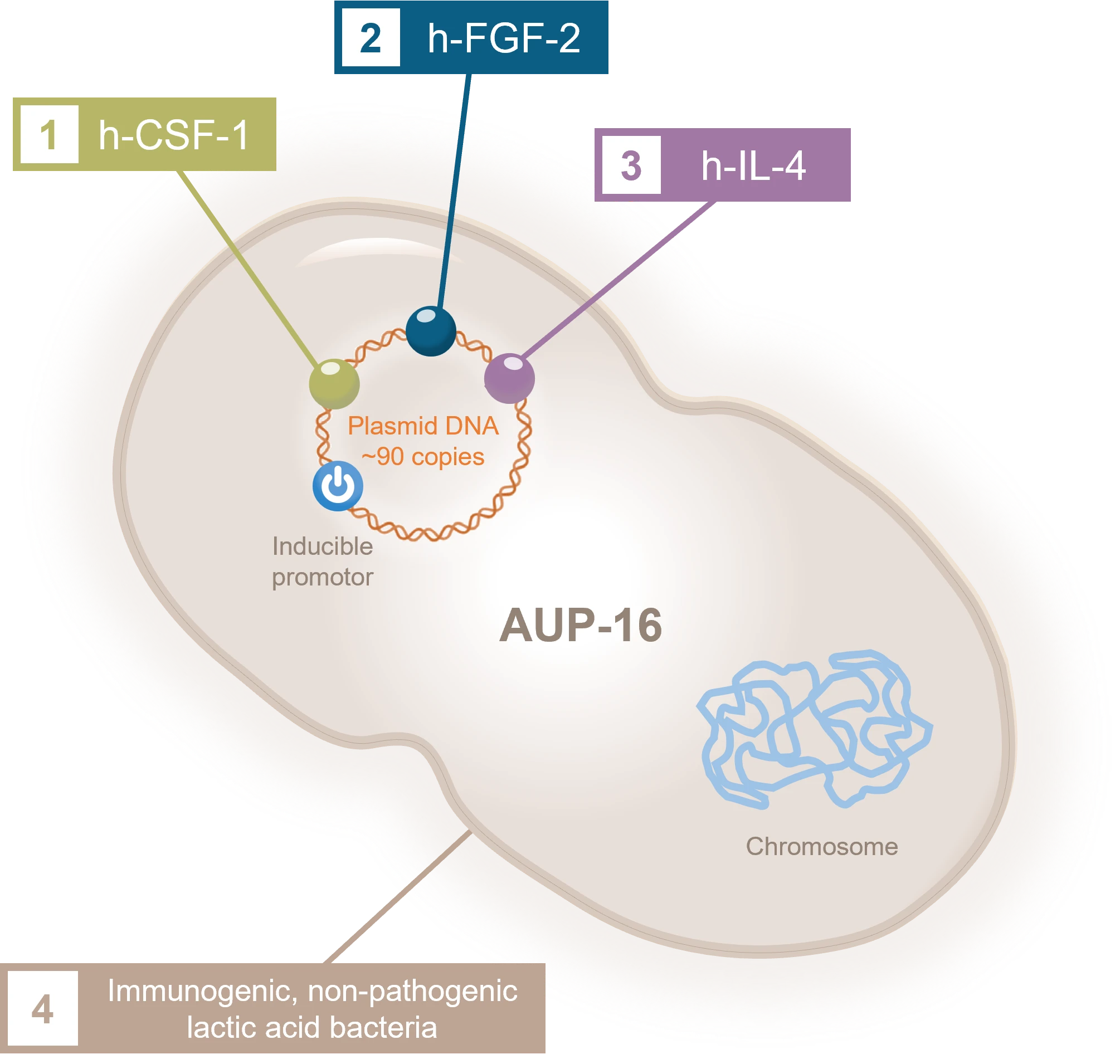
AUP-16 is a safe and effective recombinant live biotherapeutic product that features a patented active bacterial vector. AUP-16 includes four therapies in one product:
1. COLONY STIMULATING
FACTOR 1 (h-CSF-1)
A hematopoietic growth factor involved in the proliferation, differentiation, and survival of monocytes, macrophages, and bone marrow progenitor cells.
2. FIBROBLAST GROWTH FACTOR (h-FGF-2)
A clinically used growth factor and signaling protein involved in cell proliferation and tissue repair.
3. INTERLEUKIN 4
(h-IL-4)
A potent anti-inflammatory M2 cytokine that promotes regeneration.
4. LACTOCOCCUS
CREMORIS
A non-pathogenic bacteria that acts as a bioreactor in the tissue, producing human therapeutic proteins in the wound. AUP-16 takes advantage of the safe, living bioreactor to produce proteins FGF-2, IL-4 and CSF-1.
AUP-16 is a topical treatment created using a genetically modified lactococcus cremoris bacteria. AUP-16 promotes healing by producing three human therapeutic proteins that (1) improve cell activation and conversion to anti-inflammatory phenotype, (2) increase fibroblast proliferation, and (3) promote angiogenesis and granulation tissue formation.
The continuous production of the three human therapeutic proteins h-CSF-1, h-FGF-2 and h-IL-4 by the active vector lactococcus cremoris at the site of the wound, results in our unique 4-in-1 combination biological treatment which is provided as one API.
AUP-16 provides multi-therapy in one product, it is not a combination product. Thanks to its multi-therapy approach, AUP-16 is able to reduce inflammation promote proliferation and angiogenesis, and accelerate remodeling. All in one product.
Our therapy, AUP-16, is created under Good Manufacturing Practice conditions and analyzed using state-of-the-art methods to ensure identity, potency, consistency, and safety.
AUP-16 has the power to accelerate and provide complete wound healing, avoiding amputations. This remarkable advancement is ideal for patients who only achieve partial response to wound care dressings and treatment of symptoms.
As opposed to the existing standard of care, AUP-16 promotes tissue regeneration in the wound to activate healing from within.
AUP-16 RECEIVED PRIME STATUS FROM THE EUROPEAN MEDICINES AGENCY
In February 2024, the Committee for Medicinal products for Human Use (CHMP) and the European Medicines Agency (EMA) have acknowledge that non-healing Diabetic Foot Ulcers (nhDFU) are a potentially life-threatening condition with unmet need for novel therapies, and that AUP-16 has the potential to address this unmet need.
First Aurealis Therapeutics product candidate to receive PRIME designation by EMA for enhanced regulatory support facilitating the clinical development of multi-target cell and gene therapy candidate AUP-16 in the treatment of nhDFU.
PRIME designation follows positive data for 16 patients from Phase-1 first-in-human study showing a dose-dependent improvement in wound closure, 67% and 83% wound closure at 3 and 6 months respectively, demonstrating the potential to address the unmet medical need in nhDFU.
HIGHLIGHTS OF AUP-16
FOUR-IN-ONE
cell and gene therapy that stimulates endogenous tissue regeneration
BIOAVAILABLE
protein production at the site of the injury
TOPICAL APPLICATION
makes it easy to administer
SAFE
modified food-grade bacteria
LEARN ABOUT AUP-16 MODE OF ACTION
CLINICAL TRIALS IN DIABETIC FOOT ULCERS
AUP-16 phase 2 currently ongoing
PHASE 1 CLINICAL STUDY IN DFU (COMPLETED)
83% of the patients achieved complete healing. No healed ulcer recurred after 12 months follow-up.
In June 2022, we successfully completed the Phase 1 study (NCT04281992 and EudraCT 2018-003415-22) in non-healing Diabetic Foot Ulcer patients with our lead product AUP-16. The last patient last visit (LPLV) was on 20th March 2023. Results were presented during EWMA 2023 conference in Milan and will be published in 2024.
Aurealis Therapeutics technology platform works in real life: non-healing diabetic wounds heal. Clinical results: 83% of patients reached complete healing, no healed ulcer recurred after 12 months follow-up.
More details about our Phase 1 Clinical Study:
NIH National Library of Medicine record
More details about our Phase 1 Clinical Study:
EU Clinical Trials Register
PHASE 2 CLINICAL STUDY IN DFU (STATUS: DATA VERIFICATION)
AUP-16 DIAMEND Phase 2 RCT in Diabetic Foot Ulcer completed in Germany, Italy and Poland.
AUP-16 Phase 2 study (NCT06111183 and EudraCT 2022-502048-10-00) in DFU received CTA approval in May 2023; the first DFU patient was dosed in August 2023; the last patient completed treatment in October 2024. The DIAMEND study is a multi-center, patient and observer blinded, randomized, standard-of-care plus placebo-controlled study in patients with non-healing diabetic foot ulcers, conducted in Germany, Italy and Poland. Blinded evaluator data expected Q3 2025.
More details about our DIAMEND Phase 2 Clinical Study:
NIH National Library of Medicine record
More details about our DIAMEND Phase 2 Clinical Study:
EU Clinical Trials Register
SCIENTIFIC PUBLICATIONS ABOUT AUP-16
Aurealis Therapeutics had an abstract published and an e-poster presented at the European Wound Management Association (EWMA) Conference Barcelona 2025 titled: A randomized controlled Phase 2 trial to evaluate multi-factorial gene therapy AUP1602-C in the management of non-healing Diabetic Foot Ulcer. Access the abstract here and the e-poster here.
Aurealis Therapeutics published a scientific article in the Therapeutic Advances in Endocrinology and Metabolism peer-reviewed journal titled: Multi-target gene therapy AUP1602-C to improve healing and quality of life for diabetic foot ulcer patients: a phase I, open-label, dose-finding study. Access the scientific article here.
Aurealis Therapeutics presented an abstract and a poster at ISPOR Health Economic Conference titled: A Markov model to determine the cost-effectiveness of a multi-targeting bacterial gene therapy (AUP-16) at healing a Diabetic Foot Ulcer when compared to the current standard of care. Access the poster and the abstract here.
Aurealis Therapeutics presented a poster at the 14th International Symposium on Lactic Acid Bacteria titled: Multi-target bacterial gene therapy for chronic wounds and cancer. Access the poster here.
Aurealis Therapeutics gave an oral presentation at the European Wound Management Association (EWMA) Conference Milan 2023 titled: 4-in-1 live biotherapeutic Gene Therapy Medicinal Product (GTMP) accelerates healing of Diabetic Foot Ulcers (DFUs): a first-in-human, phase 1 clinical study. Access the oral presentation here.
Aurealis Therapeutics published a scientific article in the PLOS-One journal titled: 4-in-1 Combination therapy using Lactococcus lactis expressing three therapeutic proteins for the treatment of chronic non-healing wounds. Access the article here.
Aurealis Therapeutics presented a poster at the European Wound Management Association (EWMA) Conference Krakow 2018 titled: Therapeutic use of Lactococcus lactis bacteria to produce proteins locally in diseased tissues of chronic wounds. Access the oral presentation here.
SCIENTIFIC ADVISORY BOARD
diabetic foot ulcers
REFERENCES
- Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376(24):2367-75.
- Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic Foot Ulcers: A Review. JAMA. 2023;330(1):62-75.
- Chen P, Vilorio NC, Dhatariya K, Jeffcoate W, Lobmann R, McIntosh C, et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. 2023:e3644.
- Hoffstad O, Mitra N, Walsh J, Margolis DJ. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38(10):1852-7.
- Kurkipuro J, Mierau I, Wirth T, Samaranayake H, Smith W, Karkkainen HR, et al. Four in one-Combination therapy using live Lactococcus lactis expressing three therapeutic proteins for the treatment of chronic non-healing wounds. PLoS One. 2022;17(2):e0264775.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care. 1999;22(5):692-5.
- McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care. 2023;46(1):209-21.
- Meloni M, Izzo V, Giurato L, Lazaro-Martinez JL, Uccioli L. Prevalence, Clinical Aspects and Outcomes in a Large Cohort of Persons with Diabetic Foot Disease: Comparison between Neuropathic and Ischemic Ulcers. J Clin Med. 2020;9(6):1780.
- Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37(3):651-8.
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
- Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33(11):1493-8.