PARTNERING
WE BRING TRANSFORMATIVE CELL & GENE THERAPIES FOR UNMET MEDICAL NEEDS
Aurealis Therapeutics offers breakthrough solutions for massive unmet needs and major pharmaceutical markets. We are eager to meet strategic partners and investors to ensure these breakthroughs are made available to all the patients who need them.
1. EFFICIENT
Multi-therapeutic
Continuous, on-site action
2. LOW COST
Scalable
Low manufacturing cost
3. SAFE
Non-pathogenic
Local administration
4. EASY
Logistically stable
No special handling required
Chronic wounds, such as Diabetic Foot Ulcers (DFUs), Venous Leg Ulcers (VLU), and Pressure Ulcers (PU), contribute major costs to healthcare systems and societies. Just in the USA, 8.2 million patients are impacted annually by non-healing wounds and related complications. The global market for chronic wound care is estimated at 10 billion USD, with a 5% annual growth rate, revealing a need for better treatment options than the ones currently available. The market is dominated by basic wound care products, advanced dressings, cellular and tissue-based products, and medical devices. No approved drugs are marketed in USA and Europe, posing an untapped market for the first movers. The outcome of the AUP-16 Phase 1 study in DFUs makes us confident that we can change the treatment paradigm of chronic wounds.
Cancer dominates the pharmaceutical market (>160 billion USD in sales in 2021); still deadly cancers represent a massive unmet medical need. For example, according to WHO, every year 313 959 women are diagnosed and 207 252 of them die due to Ovarian Cancer. AUP-55 pre-clinical data in advanced Ovarian Cancer and Peritoneal Carcinomatosis demonstrates the flexibility and ubiquity of Aurealis Therapeutics cell and gene therapy platform in oncology.
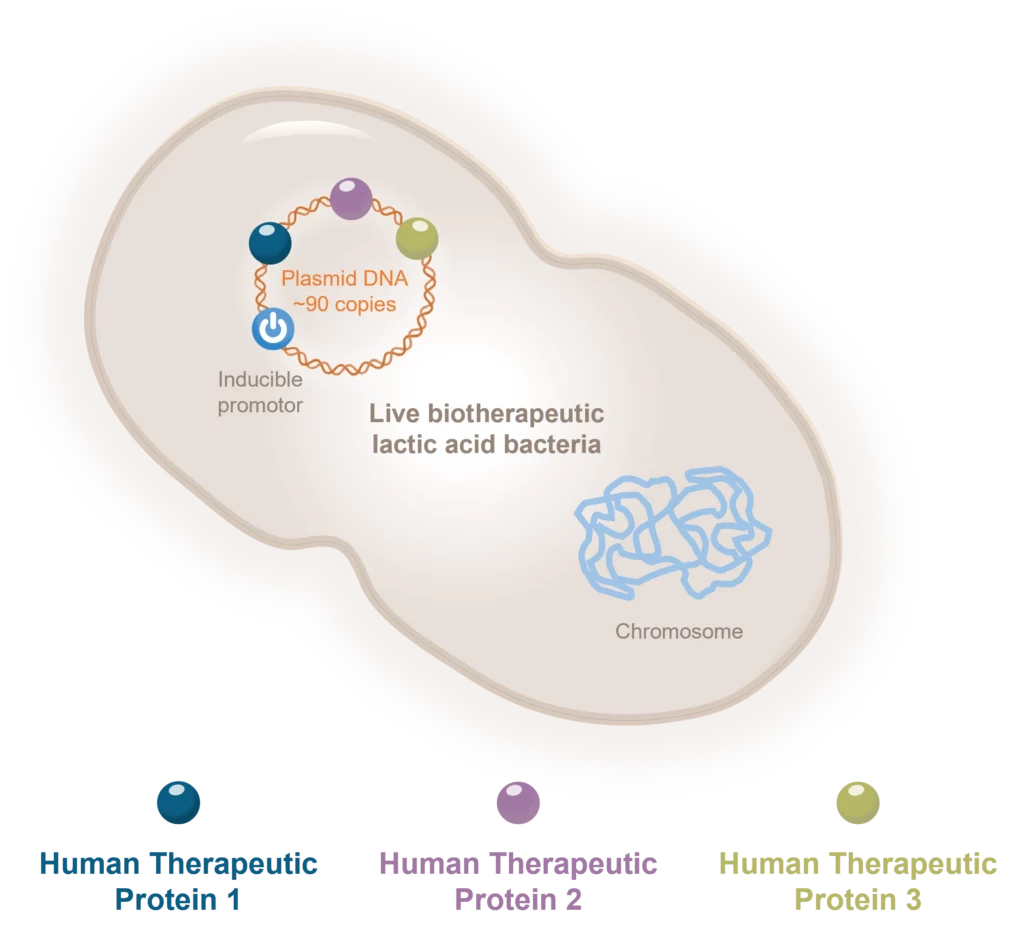
Aurealis Therapeutics is an innovative cell and gene therapy platform company offering world-first 4-in-1 therapies with superior efficacy to these unmet medical indications and market. Our disruptive technology platform clearly differentiates from other therapeutic options:
- Enables 4-in-1 targeting
- Non-pathogenic
- Controllable when administered
- Cost of goods and manufacturing scalability
- Easy to use
LICENSING & CO-DEVELOPMENT AGREEMENT IN CHINA
In January 2022, Aurealis Therapeutics signed an exclusive license and collaboration agreement with a Chinese Biotech company (Xbiome) for the clinical development and commercialization of the therapy AUP-16 for Diabetic Foot Ulcers, other chronic wounds and inflammatory diseases in Greater China.
The company acquired exclusive development and commercial rights for all human use to Aurealis Therapeutics AUP-16 in Mainland China, Hong Kong, Macao and Taiwan. Its responsibility included all clinical and other development, regulatory submissions, and commercialization of the licensed products in the licensed territory, while Aurealis Therapeutics retained full rights to AUP-16 outside of the specified territory and continued to lead the global development of the drug candidate.
In November 2023, IND approval was granted by the National Medical Products Administration (NMPA) for the AUP-16 DFU Phase 2 study to be initiated in China. Aurealis Therapeutics is now seeking a partner for AUP-16 in Greater China.
